EchoLaser is a multichannel laser system used for the ultrasound or fusionguided laser ablation of both benign and malignant lesions in the prostate, such as Benign Prostatic Hyperplasia and localized Prostate Cancer. EchoLaser was developed by the Italian Med-Tech company Elesta.
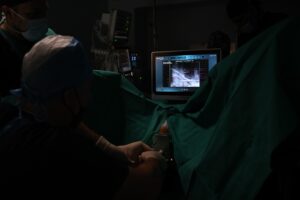
The EchoLaser procedure is micro-invasive. The laser works employing laser light transmitted by extremely fine (less than 1 mm in diameter) applicators with transperineal access, to heat the targeted lesion causing irreversible “in situ” damage without the need of tissue removal. The procedure is supported by the Echolaser Smart Interface (ESI), a dedicated software that helps the physician to correctly position of the optic fibers, raising the bar of safety and precision.
The treatment is performed in an outpatient setting under local anaesthesia in 20 to 40 minutes. The EchoLaser technology allows the preservation of the organ and its functionality. Minor risks are associated to the EchoLaser, avoiding trauma and complications related to the surgical approach. The patient comes back to daily activities just few hours after the treatment. Reduced operation time and absence of hospitalization allowing a fast patient management are additional fundamental features.
EchoLaser micro-invasive procedures have been performed for several years in Italian and International Centres of Excellence, generating a robust and growing clinical evidence.
EchoLaser treatment for the focal therapy of prostate cancer is available in Greece at Athens Medical Center Psychico Clinic with Dr. Brembos and his team. Here Dr. Brembos, for the first time worldwide, started treating prostate cancers with EchoLaser technology in combination with radiopaque gold marker (clearly visible on MRI and transrectal US) that, combined with ESI, improves the effectiveness of the lesion targeting. The markers positioned during biopsy procedure provide a tool to aim better the prostate cancer position, localize the tumour in a more accurate way and reduce the treatment time in the planning phase. Patients were treated in 30 minutes, without complications and in outpatient setting.